Titration of Antibody Protocol
Titration of Anti Body
Outcome of the Titration is a highly subjective. If the panel is for lung cells, then lungs cells must be used for titration not PBMC or Spleen which are easier to get.
Following protocol is for PBMCs but can easily be adopted for other cell types by changing the cell concentration.
- Harvest cells in staining buffer (BD, 554657). Consider 1 million cells for one tube. Calculate accordingly for harvesting. Maintain cell concentration of 1 million/100 µl of buffer.
- Add FC block (BD, 564219 or 553141 for Human or mouse) and True-Stain Monocyte Blocker (Biolegend, 426102). Use 5 µl of each for 100 µl of volume
- Incubate for 10 min at RT
- Add Ab according to the below table, considering manufacturer recommended 4 µl/test
- Take 8 µl in an Eppendorf, take 4 µl of Ab from here and add to tube 1.
- Then add 4 µl of stain buffer in the same Eppendorf, mix by pipetting, take 4 µl from here and add to tube 2
- Repeat step 6 till tube number 5. With serial dilution we can make sure the original stock does not get contaminated accidentally and same time minimizing pipetting error.
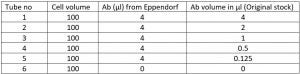
- Add stain buffer to make final volume 200 µl
- Incubate at RT for 20 minutes in dark
- Add 3 ml of cold stain buffer, quick vortex
- Spin for 5 minutes, 400g in cold
- Wash again
- Resuspend in 300 µl of stain buffer
- Run
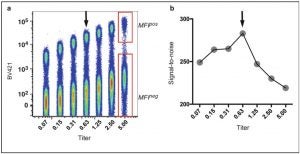
- Calculate rSD and MFI of the “negative” population and MFI of the positive population
- Calculate Stain Index using formula
(SI) = (MFI Pos – MFI Neg)/(2 x rSD Neg)
- Pick the Ab amount that has highest SI value
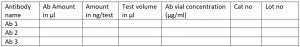
- Table for recording ab amount for future use:
- How to find antibody lot info:
Some manufacturer has lot specific concentration info in their site, most do not provide this info in their site, but they do provide when asked.
- For BD Bioscience you can use https://www.bdbiosciences.com/en-us/support/product-support/concentration-lookup
- For Biolegend you can use https://www.biolegend.com/en-us/concentration-expiration-lookup
- For Cytek you can use https://cytekbio.com/pages/certificate-of-analysis-locator?page=1