Annexin V Stain Protocol
In apoptotic cells, the membrane phospholipid phosphatidylserine (PS) is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment. Annexin V is a 35 kDa Ca2+ dependent phospholipid-binding protein that has a high affinity for PS, and with exposed PS. Annexin V may be conjugated to fluorochromes and used in flow cytometric analysis to detect cells that are in early stages of apoptosis.
It is possible to include other functional probes or surface Ab with Annexin V.
Fully stained experimental sample preparation
Once the treatment is finished, cells needed to be harvested as soon as possible and as gently as possible. This is crucial, if the harvesting is rough, it will create holes in the healthy cells. This will allow Annexin V to enter inside the cell and bind to PS present in the inner leaflet.
Assuming each treatment has 0.2 to 1 million cells.
1a. For suspension culture collect all the media and cell in a 15 ml tube. Add 3 ml cold PBS in the plate/flask and rinse thoroughly and collect in the same 15 ml tube.
1b. For adherent cell 1st collect all the media and floating cell (these are the dead cell) in a 15 ml tube. Then very gently detach the cells that are still stuck to the plate and collect them in the same tube. Do this as fast as possible.
- Spin cells at 500g for 7 min 4oC
- Decant the supernatant
- Resuspend in 100 µl of Annexin V buffer (Biolegend, 422201)
- Add 0.3 µl of Annexin V (Biologend, 640906), incubate at RT, for 10 min
- Add 200 µl of Annexin Buffer with DAPI so that final concentration become 40 ng/ml (alternately you can perform this step at core moments before loading in the machine)
- Bring sample to core in ice, protected from light.
- This is a functional assay, you need to run the samples as soon as possible
Preparation of control for the machine setup
Purpose of this controls to provide single color of FITC and DAPI so that software can deconvolute the data. You do not need to use same reagent you will use in your real experiment. You can use Staurosporine (Sigma, S4400) or Camptothecin (Sigma, C9911) to create apoptotic cell. Annexin V will only bind to apoptotic cell.
As described above collect Apoptotic cell
Single stain for Annexin V
- Get 0.2 to 1 million Apoptotic cells in 100 µl of Annexin buffer
- Add 0.3 µl of Annexin V, stain as above
- Add 200 µl fresh Annexin buffer
Single stain for DAPI
- Get 0.2 to 1 million Apoptotic cells in 100 µl of Annexin buffer
- Add 200 µl Annexin buffer with DAPI to reach 40 ng/ml final DAPI concentration
Unstained Apoptotic cell
- Get 0.2 to 1 million Apoptotic cells in 300 µl of fresh Annexin buffer
Sample you need to bring
- 3 single color sample to setup the machine
- Experimental sample as many you need
- Untreated healthy unstained cells (multiple tubes if you have more than one cell line)
- Treated Unstained cells (multiple if wish to check multiple reagents)
How to Titrate Annexin V
Amount of Annexin V needed changes with cell lines. To titrate you will need ~ 7 million Apoptotic Cell (as describe above) in 700 µl of Annexin V buffer and ~ 7 million healthy cells in 700 µl of Annexin V buffer.
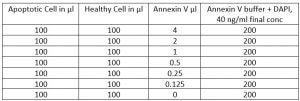
Goal is to find a Annexin amount that provides the maximum separation between Pos and negative population in Apoptotic cell and same time provides lowest nonspecific binding from Healthy cells. For adherent cells this is especially important.